Topics 2025.08.18
Were the building blocks of life made on ancient Mars?
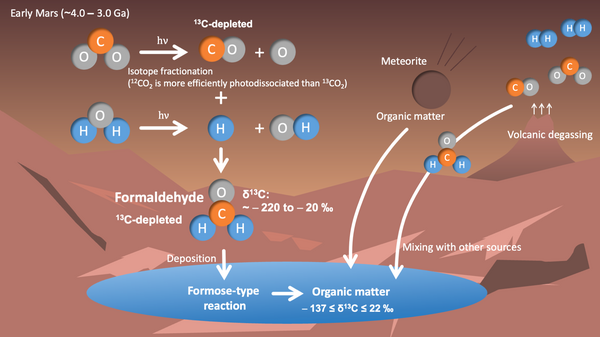
Fig. Diagram showing the processes of how organic matter was formed on early Mars. (Credit: Shungo Koyama).
Do extraterrestrial life-forms exist? Many of us have asked this question at least once. Scientists around the world are tackling it in many ways. Our team looks at Mars--the planet next door--to see whether life could have started there.
To judge whether life could ever begin, we first need to know how organic molecules (the basic "building blocks" of life) were made. One important clue is the stable carbon isotope ratio--the amounts of carbon-13 (13C) and carbon-12 (12C). Differences in this ratio can tell us how an organic substance was formed.
Mars today is cold and dry, but geological evidence suggests there was liquid water about 3-4 billion years ago. NASA's rover Curiosity found that organics in ancient Martian sediments are unusually poor in 13C (in other words, they contain more "light" carbon). It also found that the isotope ratios vary a lot from sample to sample. Why these strange values appear had remained a mystery.
We focused on a small molecule called formaldehyde (H₂CO), which can be made in the atmosphere of early Mars. After it falls to the ground, it can react in water and turn into more complex organics, including sugars, which are key materials for life. If Mars made a lot of formaldehyde for a long time, it could have supplied many of the building blocks life needs.
To test this idea, we built a Mars atmospheric evolution model by combining a photochemical model (how sunlight makes and breaks molecules) with a radiative-convective model (how energy and heat move through the air). We then estimated how the carbon isotope ratio inside atmospheric formaldehyde would have changed 3-4 billion years ago. We found that when CO₂ is broken apart by ultraviolet sunlight, a process called isotopic fractionation happens: molecules with 12C break more easily than those with 13C. As a result, the formaldehyde that forms ends up low in 13C. We also showed that this isotope ratio would change depending on factors like surface air pressure, surface reflectivity (albedo), the CO/CO₂ ratio, and the amount of hydrogen released by volcanoes.
Our results show that organics made from this formaldehyde can explain the strange carbon isotope signatures seen on Mars--especially the strong depletion of 13C. The large spread in values between samples also makes sense if Martian organics are a mixture: formaldehyde-derived organics plus other organics from volcanic gases and meteorites.
This suggests that formaldehyde helped drive organic synthesis on ancient Mars, and that molecules like sugars--the materials of life--could have formed there.
Exploration on the Martian surface is still going on, so we are learning more about what kinds of organics exist and what the environment was like when they formed. In addition, Japan's MMX (Martian Moons eXploration) mission aims to return samples that will record carbon isotopes from different times in Mars' history. Using those data, we hope to map when, where, how much, and in what molecular forms organics were made on Mars--and to get closer to the conditions that might have allowed life to begin.
Planetary Atmospheric Physics Group, Shungo Koyama
Reference
Koyama, S., Kamada, A., Furukawa, Y. et al. Atmospheric formaldehyde production on early Mars leading to a potential formation of bio-important molecules. Sci Rep 14, 2397 (2024). https://doi.org/10.1038/s41598-024-52718-9
Koyama, S., Yoshida, T., Furukawa, Y. et al. Stable carbon isotope evolution of formaldehyde on early Mars. Sci Rep 14, 21214 (2024). https://doi.org/10.1038/s41598-024-71301-w